Among the constant stream of new injectables and “skin boosters,” few innovations have drawn as much genuine scientific interest as polynucleotides. They are not presented as instant transformations or filler replacements, but as something quieter and more ambitious: molecules that communicate directly with the skin’s biology and support repair from within.
To understand why serious clinics have adopted them, and why patients with intelligent questions should pay attention, it is necessary to move past the marketing vocabulary and look at what polynucleotides are actually doing at the molecular level.
What polynucleotides are

Polynucleotides are long chains of nucleotides, the same fundamental units that make up DNA and RNA. In aesthetic medicine, they are usually highly purified fragments derived from regulated biological sources, processed so that proteins and immunogenic components are removed, leaving stable strands that can interact safely with the skin.
Unlike hyaluronic acid fillers, they are not designed to sit in the tissue and hold space. Their purpose is not to alter facial shape or create volume. Instead, they act as bio-stimulatory molecules. Once introduced into the dermis, they participate in cell signalling and help create an environment in which the body’s own regenerative processes are more active and more efficient.
How they influence fibroblasts and the extracellular matrix
The fibroblast sits at the centre of meaningful skin rejuvenation. This is the cell responsible for producing collagen, elastin and glycosaminoglycans, the elements that maintain strength and elasticity. With age, fibroblasts slow down; with chronic UV exposure, they can even become senescent, releasing enzymes that degrade surrounding tissue.
Polynucleotides help to recalibrate that balance. In laboratory studies, exposure to specific polynucleotide preparations increases fibroblast metabolic activity. These cells produce more type I and type III collagen and organise it more coherently. At the same time, expression of matrix metalloproteinases, the enzymes responsible for collagen degradation, is reduced. The result is not an artificial scaffold but a more robust, better maintained dermal matrix that the skin has built for itself.
This is one of the reasons polynucleotides are described as regenerative rather than corrective. They are not imposing an external structure; they are reminding an existing system how to function more like its younger self.
Effects on blood flow, inflammation and oxidative stress
Good quality skin is well-supplied, well-oxygenated and able to modulate inflammation. Polynucleotides appear to support all three of these processes. Several mechanistic studies suggest that polynucleotide treatments increase the expression of growth factors associated with angiogenesis, including vascular endothelial growth factor. In practical terms, this can improve microcirculation in the dermis, supporting more effective delivery of nutrients and clearance of waste products. Skin that is better perfused tends to heal more reliably.
In parallel, polynucleotides have been shown to buffer oxidative stress. Modern skin exists in a constant stream of ultraviolet radiation, particulate pollution and lifestyle-related reactive oxygen species. These factors damage DNA, lipids and proteins and drive the chronic low-grade inflammation that accelerates ageing. Polynucleotides, by providing nucleic acid fragments and engaging specific receptors, contribute to DNA repair pathways and reduce inflammatory signalling. The local environment shifts away from breakdown and towards maintenance.
Hydration and the dermal microenvironment
One of the more tangible early effects of polynucleotide treatment is improved hydration. The formulations are hydrophilic, meaning they attract and hold water within the dermal layers. However, this is not simple swelling. The water-binding effect supports enzymatic reactions and cellular communication that depend on a stable aqueous environment.
A hydrated dermal matrix is more than comfortable. It is a prerequisite for efficient cell migration, collagen assembly and barrier support. The hydrated matrix also supports lymphatic flow and waste clearance, often explaining why patients describe the skin as calmer and less congested after a series of treatments.
When the groundwork is in place, the skin is better prepared to respond to other interventions, from light-based therapies to fractional lasers. This is why polynucleotides are increasingly used not only as stand-alone treatments, but as part of structured protocols that prepare and maintain the skin around more intensive procedures.
Clinical outcomes without theatrics
For patients, the important question is what all of this translates to in the mirror.
Polynucleotides do not deliver a sudden, dramatic change in contour. Instead, over a period of weeks to months, the skin begins to look clearer, more even and subtly firmer. Fine lines soften, texture becomes smoother, and there is a shift in the way light reflects from the surface. These changes correspond to what the underlying biology predicts: healthier collagen, improved hydration and a quieter inflammatory background.
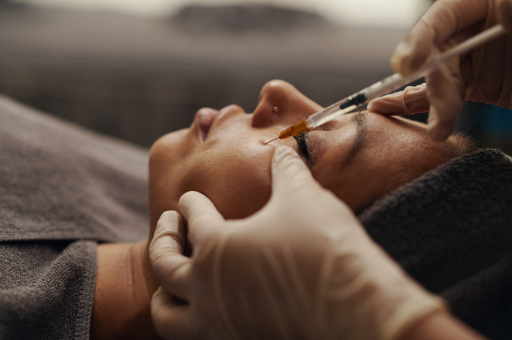
A typical treatment course involves a series of sessions separated by several weeks. In each session small volumes are placed intradermally with fine needles or a cannula. Mild swelling, redness or papules settle quickly. Because the material is highly purified and biocompatible, adverse reactions are rare when the correct products and techniques are used. Maintenance sessions a few times a year can sustain the effect by keeping fibroblasts engaged.
The pace is deliberate. This is not an instant result for an event tomorrow, but a strategy for skin that should function better in six months and a year.
Polynucleotides for skin ageing
In the context of intrinsic and photo-induced ageing, polynucleotides answer a problem that topical skincare and traditional injectables alone cannot fully solve.
Topical antioxidants and retinoids act primarily at or near the epidermis. Hyaluronic acid fillers address volume loss and contour. Neither directly recalibrates dermal cell signalling in the way polynucleotides aim to. By enhancing fibroblast activity, protecting against oxidative DNA damage and supporting microvasculature, polynucleotides address the cellular exhaustion that sits beneath fine lines and loss of firmness.
The ideal candidate is not necessarily the patient seeking maximal lifting. It is the person who has early laxity, crepey texture, dullness after sun exposure or a sense that their skin no longer bounces back from stress. In these cases, the most elegant intervention is often to restore the skin’s behaviour rather than to disguise its outcome.
Polynucleotides in acne scarring
Acne scars are structural reminders of inflammation. They represent areas where collagen architecture has been lost or replaced with disorganised fibrous tissue. Traditional scar revision focuses correctly on mechanical and energy-based methods: subcision, coring, fractional lasers, radiofrequency. These break down tethering and stimulate new collagen.
Polynucleotides enhance this process from the inside. By increasing fibroblast activity and improving vascular support, they can help the skin lay down better quality collagen in response to controlled injury. Used in the right sequence, they do not replace lasers but make the healing phase more productive.
Their use is particularly valuable in darker or reactive skin types, where aggressive resurfacing carries a higher risk of pigmentation. By improving dermal quality before and between treatments, polynucleotides can reduce the likelihood of post-inflammatory pigment change and enhance uniformity of tone as scars remodel.
In practice, polynucleotides can be introduced between fractional treatments or subcision to support organised remodelling rather than chaotic repair. The goal is a smoother transition between untreated and previously scarred skin, achieved through combined structural and biological intervention.
Priming the skin before laser and other devices
Energy-based devices are powerful tools for texture, tone and tightening, but outcomes depend heavily on the condition of the skin at baseline. A dehydrated, inflamed or nutritionally compromised dermis responds less predictably and recovers more slowly.
Using polynucleotides as a priming step acknowledges that reality. When the dermis is better hydrated, more vascularised and populated with metabolically active fibroblasts, the response to fractional lasers, ultrasound or broadband light is typically more efficient. Collagen induction is stronger, downtime can be smoother and the risk of post-inflammatory pigment change may be reduced in appropriate candidates. Patients often notice faster resolution of redness and swelling when this priming step is included, reflecting more efficient tissue recovery.
At Self London, this principle is used deliberately. Patients who are likely to benefit from resurfacing or tightening are often guided through a preparatory phase to ensure that barrier function, dermal hydration and cellular health are optimised beforehand. Polynucleotides are one of the tools that allow this preparatory work to be both targeted and science-based.
Where polynucleotides sit among other injectables
It is important to distinguish polynucleotides clearly from familiar treatments. Fillers restore shape. Botulinum toxin adjusts muscular activity. Profound resurfacing and collagen induction come from lasers and radiofrequency.
Polynucleotides belong to a separate category: regenerative injectables that aim to improve tissue quality. They do not correct significant volume loss, cannot substitute for a surgical lift and will not erase deep etched lines in isolation. Presenting them as such undermines trust. Their strength lies in supporting healthier skin architecture so that, where other interventions are appropriate, they can be used more sparingly and more successfully.
This also means polynucleotides are often well suited to patients who actively want to avoid an obvious treated look. Results are subtle by design, where the skin looks better, not different.
Safety, provenance and intelligent caution
The reassurance around polynucleotides comes from both mechanism and experience, but intelligent caution is still warranted. Products should be sourced from reputable manufacturers using high-grade purification and supported by preclinical and clinical data. The injection technique must respect anatomy and depth. Treatment plans should be individualised rather than driven by trends.
There are still questions being refined: optimal dosing schedules, best combinations with other procedures, and the durability of effect across different age groups and skin types. This is normal in any evolving field. A responsible clinic acknowledges what is known and what is emerging, rather than overstating certainty.
How Self London uses polynucleotides
At Self London, treatment protocols are always designed around clinical logic and biological coherence. Polynucleotides are used where they offer a genuine advantage – for early to moderate skin ageing, to restore texture, elasticity and luminosity as part of a broader plan that always includes barrier repair and sun protection.
For those with acne scarring, they are integrated into scar revision protocols to enhance the quality of collagen laid down after mechanical and laser-based treatments. For individuals about to undergo fractional laser, ultrasound tightening or other energy-based procedures, they are used to prime the dermis so that healing is more controlled and results are more robust.
In every case, the decision to use polynucleotides is made in consultation with a doctor, based on anatomy, indications and long-term strategy. They are not presented as a miracle or a shortcut, but as one of the most precise tools currently available for encouraging skin to repair and regenerate in a way that is consistent with its own biology.
A more serious standard for “skin boosters”
If there is a single reason polynucleotides resonate with a discerning audience, it is this: they treat the skin as an organ rather than an accessory. They fit a modern standard of care in which interventions are selected not for their novelty, but for their coherence with physiology. By improving the conditions in which cells function, they support every other considered choice a patient might make, from high-factor sunscreen to carefully calibrated lasers.
For those who value evidence over embellishment and seek treatments grounded in real biology, polynucleotides represent quiet progress, the kind of medicine that earns trust not through spectacle, but through results that last.